Emission Spectroscopy
“Emission spectroscopy is a spectroscopic technique, investigates the photon’s wavelength when emitted by atoms or molecule during the transition of excited state to a lower energy state.”
So, emission spectroscopy is an important technique to study. Still, before enriching about this topic, we must know some of the basic concepts in brief, such as Spectroscopy, Spectroscope or Spectrometer, wavelengths of photons, atomic spectroscopy emission line, and emission spectra, etc. Then this topic, “Emission Spectroscopy” will be interesting and easy to understand.
Emission spectroscopy is popularly termed as optical emission spectroscopy because of the light nature of what is being emitted.
What is “Spectroscopy” and “Spectrometry”?
Spectroscopy:
“Spectroscopy the investigation of interactions amongst matters with various types of electromagnetic radiation;”
Usually, it is utilized for different measurements and quantitative analysis; the term “Spectrometry” is used.
What is Spectroscope or a Spectrometer?
A spectrometer or a spectroscope is an instrument that is used for separating the components of light, which have different wavelengths.
Basic Principle of Spectroscopic Technique:
The basic principle shared by all spectroscopic techniques is to analyze a beam of electromagnetic radiation on a sample and observe how it responds to such a stimulus.
Spectroscopy Types
Atomic spectroscopy techniques are as follows:
- AAS- Atomic Absorption Spectroscopy
- AFS- Atomic Fluorescence Spectroscopy
- AES- Atomic Emission Spectroscopy
- XRF- X-Ray Fluorescence
- MS-Mass Spectroscopy

In most of these methods (i.e., AAS, AFS, and AES), the phenomena of interactions between ultraviolet light and the valence electron of free gas atoms have been exploited. In the X-ray fluorescence, the high-energy charged particles will collide with intra-shell electrons of an atom, initiate the subsequent photon emission during the transitions. For inorganic mass spectroscopy, ionized analyze atoms are usually detached in the applied magnetic field according to (m/z) mass to charge ratio, and utilized for further investigation using this basic phenomenon.
What is meant by atomic emission?
As we know, the emission is the production and discharge of something, especially gas or radiation. The spectrum is the distinctive feature of the matter or emitting element or substance and the type of excitation to which it is subjected to compare the absorption spectrum. The atomic emission can be utilized to analyze a free gaseous atom. This is the most common method for plasma, arc, and flames, each of which is useful for a solution or liquid samples—the aggregate of energy functions as the excitation source in this method.
Atomic Spectroscopy:
“Atomic spectroscopy is related to electromagnetic radiation absorption and emission by atoms. Since unique elements have characteristic (signature) spectra, atomic spectroscopy, specifically the electromagnetic spectrum or mass spectrum, is applied to determine elemental compositions.”
Why is atomic spectroscopy important?
Spectroscopy plays a substantial part in various analytical methods that contribute information on elemental concentrations and isotopes ratios.
- It is used to analyze protons or X-ray photons or particle-induced X-ray emission in X-ray fluorescence and Energy-dispersive X-ray spectroscopy. So atomic spectroscopy is an important technique used in fluorescence spectroscopy by exploiting the interaction with electromagnetic radiation.
Atomic Spectrum:
The atomic spectrum is the range of characteristic frequencies of electromagnetic radiation that are absorbed and emitted by an atom. The atomic spectrum provides a visual overview of these orbits of electrons around an atom.
An electron can jump from a fixed orbital to the following one as follows:
- The electron should absorb a photon of a particular frequency; When an electron jumps into have higher energy.
- If it jumps to lower energy, then it has to emit a photon of a particular frequency.
- The emission spectrum of each chemical element is largely responsible for the color of things and is unique. Atomic spectra can be analyzed to find out the composition of objects.
- The explanation of this phenomenon is critical to the progress of quantum mechanics.
Atomic emission spectroscopy
“Atomic emission spectroscopy (AES) is a technique of analysis that uses the intensity of light emitted from plasma, arc, spark, and flame at a particular wavelength to determine the quantity of an element in a sample.”
Atomic spectroscopy contains lots of analytical methods used to compute the elemental composition (it could be liquid, gas, or solid) by detecting the electromagnetic emission spectra, emission intensity, or mass spectrum of that sample. Element concentrations of a million (ppm) or one billion component (ppb) of this sample could also be discovered, so it could be used for vacuum analysis. There are different types of mass spectroscopy, spectroscopy, emission, absorption, and fluorescence techniques. Because each has its strengths and constraints, the determination of a suitable technique calls for a fundamental comprehension of each method. This topic is intended to offer the emission spectroscopy methods only, though.
It is a chemical analysis system that employs the intensity of light, emission intensity generated from a hot gas flame, arc, plasma, or discharge at a specific wavelength to ascertain the number of a component in a sample. While the emitted light level is proportional to the number of atoms of this component, the wavelength of the spectral line at the emission spectrum provides this component’s identity. Several procedures may excite the sample.
Method to generate Emission Spectrum and Absorption Spectrum

What is Emission Spectra or Emission Spectrum?
“The emission spectrum of an element or chemical compound is that the range of frequencies of electromagnetic radiation emitted because of an atom or molecule jump or transitioning from a higher energy state to a lower energy state.”
The emission line or spectral line is either bright or dark in an otherwise continuous or uniform spectrum, leading to emission or absorption of light in a narrow frequency range, compared with the standard elemental frequencies. These emission spectral lines are utilized to recognize atoms and molecules by comparing them with the standard elemental frequencies.
Sample of Emission Spectra:

The emission spectrum of iron (Fe).
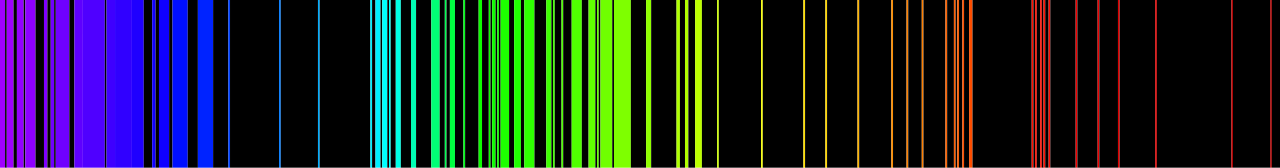
Image credit :
Nilda, Public domain, via Wikimedia Commons
Type of Atomic emission spectroscopy:
- · Inductively coupled plasma atomic Atomic emission spectroscopy.
- · Spark or arc atomic Atomic emission spectroscopy.
- · Flame based atomic Atomic emission spectroscopy.
Inductively coupled plasma Atomic emission spectroscopy:
Inductively coupled plasma atomic emission spectroscopic technique (ICP-AES) utilizes an inductively coupled plasma to make excited atoms and ions will emit electromagnetic radiation at different characteristic wavelengths of a specific component. The benefits of inductively coupled plasma atomic emission spectroscopic technique have the limitation of multi-element capability, low chemical interference, and a stable and reproducible signal.
Cons are spectral interference (many emission lines), price and operating expense, and the fact that samples normally need to maintain a liquid remedy.
The atomic emission spectroscopic technique is a chemical investigation scheme that employs the intensity of light generated from a hot gas flame, arc, plasma, or discharge at a specific wavelength to ascertain the number of a substance or component. While the level of the emitted light is proportionate to the number of atoms of this component, the spectral line’s wavelength at the emission spectrum provides this component’s identity. Several procedures may excite the sample.
Spark or arc atomic emission spectroscopy:
“A type of atomic emission spectrometry in which the sample is excited by an arc or spark between two electrodes.”
Spark or arc atomic emission spectroscopy can be utilized for the evaluation of metallic components in solid samples. For non-conductive materials, the sample is the mixture with graphite powder to make it perceptible. In conventional arc spectroscopy methods, a sample of the sound was generally ground up and ruined via evaluation processing. The excited atoms emit light at characteristic wavelengths, which might be dispersed with a monochromator and detected.
In an earlier age, the arc or spark technique was not adequately controlled; the evaluation for those components in the sample has been qualitative only. But, modern spark resources with discharge control has become highly qualitative. Both qualitative and quantitative spark evaluation are commonly used to manufacture quality management from foundry and metal casting centers.
Flame atomic emission spectroscopy:
A sample of the substance is mixed or brought into (by using a small loop of platinum or specific wire) to the flame of gas, or sprayed solution, or directly into flame or fire. Flame evaporates the sample solvent by the existing heat produced and breaks intramolecular bonds to produce free atoms. This energy will excite the atom, especially electrons, too excited electronic states which emit light when they jump back in the ground electronic state. Each element emits light or photon at a predefined characteristic wavelength, that is dispersed using a prism or grating apparatus and finally observed in the spectrometer.
Frequent use of this emission measurement with flame and spark is standardized for alkaline metals to get pharmaceutical analytics.
What is the difference between atomic absorption spectroscopy (AAS) and atomic emission spectroscopy (AES)?
- Atomic absorption spectroscopy (AAS) and atomic emission spectroscopic (AES) technique is a Spectro-analytical process for the quantitative analysis of compound components employing the absorption of optical radiation (light) free of electrons from the gaseous condition.
- In AAS-atomic absorption spectroscopy, when monochromatic light is bombarded via the substance that the electrons absorb energy, the absorption level is listed. In the atomic emission spectroscopic (AES) technique, the sample that gets atomized in the flame then absorbs the electrons’ energy and becomes excited.
- The information about excitation and emission spectra (or excitation spectra and emission intensity decay), energy level allows access to information about the distributions in both grounds- and excited states.
- The use of different light source and excitation source is method specific.
Application of Emission Spectroscopy:
- A laboratory-based hard x-ray monochromator is used for high-resolution applications exploiting the X-ray emission spectroscopy.
- A standard application is also near edge structure measurements as the atoms decay to the ground stage, exploiting X-ray absorption. The emitted radiation usually passes through the monochromator used to isolate the specific characteristic wavelength for this specific analysis.
- Emission spectroscopy in AES or Atomic-emission spectroscopy generally exploits the quantifiable optical emission measurement starting as of excited atoms to assess concentration and its emission spectra. Extra specifics concerning the electronic and geometric structure of transition metals could also be investigated and analyzed.
- Spectroscopic measures based on emission spectrum and nonlinear x-ray spectroscopy are used to analyze a different type of transition such as metal compounds in inorganic chemistry, characterization of catalysis, and materials science application.
Also Read:

I am Subrata, Ph.D. in Engineering, more specifically interested in Nuclear and Energy science related domains. I have multi-domain experience starting from Service Engineer for electronics drives and micro-controller to specialized R&D work. I have worked on various projects, including nuclear fission, fusion to solar photovoltaics, heater design, and other projects. I have a keen interest in the science domain, energy, electronics and instrumentation, and industrial automation, primarily because of the wide range of stimulating problems inherited to this field, and every day it’s changing with industrial demand. Our aim here is to exemplify these unconventional, complex science subjects in an easy and understandable to the point manner.

Hi Fellow Reader,
We're a small team at Techiescience, working hard among the big players. If you like what you see, please share our content on social media. Your support makes a big difference. Thank you!