Electron density refers to the measure of the probability of finding an electron in a particular region of an atom or molecule. It provides valuable insights into the behavior and properties of atoms and molecules. The electron density distribution determines various characteristics such as bond lengths, bond angles, and molecular shape. Understanding electron density is crucial in fields like chemistry, physics, and materials science, as it helps in predicting chemical reactivity, molecular interactions, and the overall behavior of matter.
Key Takeaways:
| Electron Density | |
|---|---|
| 1 | Measures the probability of finding an electron in a specific region |
| 2 | Determines bond lengths, bond angles, and molecular shape |
| 3 | Crucial in predicting chemical reactivity and molecular interactions |
| 4 | Provides insights into the behavior and properties of atoms and molecules |
Understanding Electron Density
Electron Density Definition
In the realm of quantum mechanics, electron density refers to the distribution of electrons within an atom or molecule. It provides valuable insights into the behavior and properties of atoms, as well as their interactions with other atoms to form chemical bonds. Electron density is a fundamental concept in quantum chemistry and plays a crucial role in understanding the electronic structure of matter.
Electron Density Meaning
Electron density can be thought of as a measure of the probability of finding an electron at a particular location in space. It is represented by a three-dimensional function known as the wave function, which is derived from the solutions to the Schrödinger equation. The wave function describes the behavior of electrons in terms of their energy, position, and spin.
The electron density is highest in regions where the probability of finding an electron is greatest. This information is often visualized using electron density maps, which provide a graphical representation of the electron distribution within an atom or molecule. These maps help scientists understand the arrangement of electrons and their involvement in chemical bonding.
Electron Density in Physics: Formula and Explanation
Mathematically, electron density (ρ) is defined as the probability density function squared (|ψ|^2), where ψ represents the wave function. The electron density can be calculated for individual atoms, molecules, or even larger systems.
The electron density is influenced by various factors, including the number of electrons, their distribution, and the shape of the atomic orbitals. It is particularly important in determining the behavior of valence electrons, which are responsible for chemical bonding and the formation of molecules.
Quantum chemistry utilizes electron density to study the electronic structure of atoms and molecules. Density functional theory (DFT) is a widely used computational method that relies on electron density calculations to predict and analyze chemical properties. By understanding the electron density, scientists can gain insights into the stability, reactivity, and overall behavior of chemical systems.
Importance and Applications of Electron Density

Why is Electron Density Important?
Electron density refers to the distribution of electrons within an atom or molecule. It plays a crucial role in understanding various aspects of chemistry and physics. Here are some reasons why electron density is important:
Chemical Bonding: Electron density is directly related to chemical bonding. It helps us understand how atoms come together to form molecules and how they interact with each other. By analyzing the electron density, we can determine the type of bonding present, such as covalent, ionic, or metallic.
Reactivity: The reactivity of a molecule is determined by its electron density. Regions of high electron density are more likely to undergo chemical reactions. By studying the electron density, we can predict the reactivity of a molecule and understand how it will interact with other substances.
Molecular Structure: Electron density provides valuable information about the shape and structure of molecules. It helps us determine the bond lengths, bond angles, and overall geometry of a molecule. This information is crucial for understanding the properties and behavior of different compounds.
Electronegativity: Electron density is closely related to electronegativity, which is the ability of an atom to attract electrons towards itself. By analyzing the electron density, we can determine the electronegativity of different atoms within a molecule. This knowledge is essential for understanding the polarity of molecules and their interactions with other substances.
How Electron Density Affects Polarity and Reactivity
Electron density plays a significant role in determining the polarity and reactivity of molecules. Here’s how it affects these properties:
Polarity: The distribution of electron density within a molecule determines its polarity. If the electron density is unevenly distributed, with one region having a higher electron density than another, the molecule will be polar. This polarity affects the molecule’s physical and chemical properties, such as solubility, boiling point, and intermolecular forces.
Reactivity: Regions of high electron density are more likely to undergo chemical reactions. This is because electrons are negatively charged and are attracted to positively charged species. Molecules with high electron density are more reactive and tend to participate in chemical reactions, such as bond formation or breaking.
Nucleophilicity and Electrophilicity: Electron density also determines the nucleophilicity and electrophilicity of molecules. Nucleophiles are species with high electron density that can donate electrons to form new bonds. Electrophiles, on the other hand, are species with low electron density that can accept electrons to form new bonds. Understanding electron density helps us identify nucleophiles and electrophiles in chemical reactions.
Electron Density and its Relation to Electronegativity
Electron density is closely related to electronegativity, which is the ability of an atom to attract electrons towards itself. Here’s how electron density and electronegativity are connected:
Electron Distribution: Electronegative atoms have a higher electron density around them due to their strong attraction for electrons. This creates regions of high electron density near electronegative atoms and regions of low electron density near less electronegative atoms.
Polar Bonds: The difference in electron density between two bonded atoms determines the polarity of the bond. If the electron density is higher around one atom, it will have a partial negative charge, while the other atom will have a partial positive charge. This creates a polar bond.
Electronegativity Trends: By analyzing the electron density and electronegativity of different elements, we can observe trends in the periodic table. Electronegativity generally increases from left to right across a period and decreases from top to bottom within a group. These trends can be explained by the electron distribution and the resulting electron density around different atoms.
Electron Density in Different Elements and Compounds

Electron density refers to the distribution of electrons within an atom or molecule. It provides valuable insights into the behavior and properties of different elements and compounds. By understanding electron density, scientists can gain a deeper understanding of the atomic structure, chemical bonding, and various other aspects of quantum chemistry.
Electron Density of Water
Water, with its molecular formula H2O, is a compound that consists of two hydrogen atoms and one oxygen atom. The electron density of water is determined by the arrangement of its constituent atoms and their electron configurations. In water, the oxygen atom has a higher electron density compared to the hydrogen atoms due to its larger number of valence electrons. This difference in electron density contributes to the unique properties of water, such as its ability to form hydrogen bonds and exhibit a high boiling point.
Electron Density of Copper
Copper is an element with the atomic number 29 and the symbol Cu. It is widely used in various industries due to its excellent electrical conductivity and malleability. The electron density of copper is influenced by its atomic structure and electron configuration. Copper has a partially filled 3d orbital, which contributes to its high electron density. This dense electron cloud surrounding the copper nucleus allows for efficient electron transfer, making copper an excellent conductor of electricity.
Electron Density of Gold
Gold, symbolized by Au and atomic number 79, is a highly valued precious metal known for its lustrous appearance and resistance to corrosion. The electron density of gold is influenced by its atomic structure and electron configuration. Gold has a partially filled 5d orbital, which contributes to its high electron density. This dense electron cloud surrounding the gold nucleus gives rise to its characteristic properties, such as its ability to reflect light and its malleability.
Electron Density of Aluminum
Aluminum, symbolized by Al and atomic number 13, is a lightweight metal widely used in various industries due to its low density and high strength-to-weight ratio. The electron density of aluminum is influenced by its atomic structure and electron configuration. Aluminum has a partially filled 3p orbital, which contributes to its electron density. This electron distribution allows aluminum to form strong metallic bonds, making it suitable for applications that require strength and durability.
Electron Density Distribution and States
What is Electron Density Distribution?
In the realm of quantum mechanics, the concept of electron density distribution plays a crucial role in understanding the electronic structure of atoms and molecules. It refers to the spatial distribution of electrons within an atom or molecule. The electron density distribution provides valuable insights into the probability of finding an electron at a particular location.
The electron density distribution is determined by various factors, including the electron configuration, molecular orbital theory, and chemical bonding. It is described mathematically by the wave function, which is a solution to the Schrödinger equation. The wave function provides information about the probability of finding an electron in a specific region of space.
Electron Density of States
The electron density of states (DOS) is a concept used to describe the distribution of energy levels available to electrons in a material. It provides information about the number of electron states per unit energy range. The DOS is an important quantity in the field of quantum chemistry and solid-state physics.
The DOS is influenced by the electronic structure of a material, which is determined by the arrangement of atoms and their respective energy levels. The DOS can be experimentally determined or calculated using theoretical models such as density functional theory. By analyzing the DOS, scientists can gain insights into the electronic properties and behavior of materials.
Where is Electron Density Highest?
The electron density distribution is not uniform throughout an atom or molecule. Certain regions tend to have a higher electron density than others. The electron density is highest in regions where the probability of finding an electron is greater according to the wave function.
In atoms, the electron density is highest near the atomic nucleus, particularly for the innermost electrons. This is due to the attractive force between the negatively charged electrons and the positively charged nucleus. The electron density decreases as we move further away from the nucleus.
In molecules, the electron density is influenced by the arrangement of atoms and the types of chemical bonds present. For example, in covalent bonds, the electron density is concentrated between the bonded atoms. In ionic bonds, the electron density is shifted towards the more electronegative atom.
Understanding the electron density distribution and states is crucial for comprehending the behavior of atoms and molecules. It allows us to predict and explain various properties, including reactivity, stability, and spectroscopic behavior. By studying the electron density, scientists can delve deeper into the intricacies of atomic structure and unravel the mysteries of quantum physics.
Electron Spin and Charge Density
What is Electron Spin Density?
In the field of quantum mechanics, electron spin density refers to the distribution of electron spins within an atom or molecule. It provides information about the spatial arrangement of electrons with different spin orientations. The concept of electron spin arises from the intrinsic property of electrons to possess a magnetic moment, which can be either “spin up” or “spin down” along a particular axis.
To understand electron spin density, we need to delve into the realm of atomic orbitals and electron configuration. According to molecular orbital theory, electrons are described by wave functions that satisfy the Schroedinger equation. These wave functions, also known as orbitals, represent the probability distribution of finding an electron in a particular region of space. The Pauli exclusion principle states that no two electrons can have the same set of quantum numbers, including spin.
The electron spin density can be visualized as a cloud-like representation of the electron distribution around the atomic nucleus. It provides insights into the arrangement of valence electrons and their involvement in chemical bonding. By studying the electron spin density, scientists can gain a deeper understanding of the electronic structure and properties of atoms and molecules.
What is Electron Charge Density?
In quantum chemistry, electron charge density refers to the spatial distribution of electron charge within an atom or molecule. It provides information about the probability of finding an electron at a specific location. The electron charge density is closely related to the electron spin density, as both are fundamental aspects of the electron cloud surrounding the atomic nucleus.
The concept of electron charge density arises from the wave nature of electrons and the probabilistic interpretation of their behavior. According to the principles of quantum physics, the wave function of an electron describes the probability amplitude of finding the electron at a given position. The square of the wave function, known as the electron probability density, represents the likelihood of finding an electron in a small volume of space.
By integrating the electron probability density over a particular region, we can obtain the electron charge density. This information is crucial for understanding various properties of atoms and molecules, such as their reactivity, polarity, and intermolecular interactions. Density functional theory, a widely used computational method in quantum chemistry, relies on the electron charge density to calculate the electronic structure and energetics of molecules.
Calculating and Determining Electron Density
Electron density is a fundamental concept in quantum mechanics and plays a crucial role in understanding the behavior of atoms and molecules. It refers to the distribution of electrons within an atom or a molecule, providing valuable information about its electronic structure and chemical properties.
How to Find Electron Density
To find the electron density of an atom or molecule, we need to consider its electronic configuration. The electronic configuration is determined by the arrangement of electrons in atomic orbitals, which are regions of space where electrons are most likely to be found. The distribution of electrons in these orbitals gives rise to the electron cloud surrounding the atomic nucleus.
In quantum mechanics, the wave function describes the behavior of electrons and is governed by the Schrödinger equation. The square of the wave function, known as the electron probability density, gives us the electron density. It represents the likelihood of finding an electron at a particular point in space.
How is Electron Density Calculated?
The calculation of electron density involves complex mathematical equations and requires the use of advanced techniques in quantum chemistry. One commonly used method is density functional theory (DFT), which approximates the electron density by solving the Schrödinger equation for the system under consideration.
DFT takes into account the interactions between electrons and the atomic nucleus, as well as the electron-electron repulsion described by the Pauli exclusion principle. By solving the equations numerically, it is possible to obtain an accurate representation of the electron density distribution.
Electron Density Formula and Equation
The electron density formula can be expressed as the square of the wave function, denoted by |Ψ|^2. Mathematically, it is given by:
Electron Density = |Ψ|^2
Here, |Ψ| represents the wave function, which depends on the quantum numbers that describe the electronic structure of the system. These quantum numbers include the principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number.
Electron Density in Chemistry and Physics Models
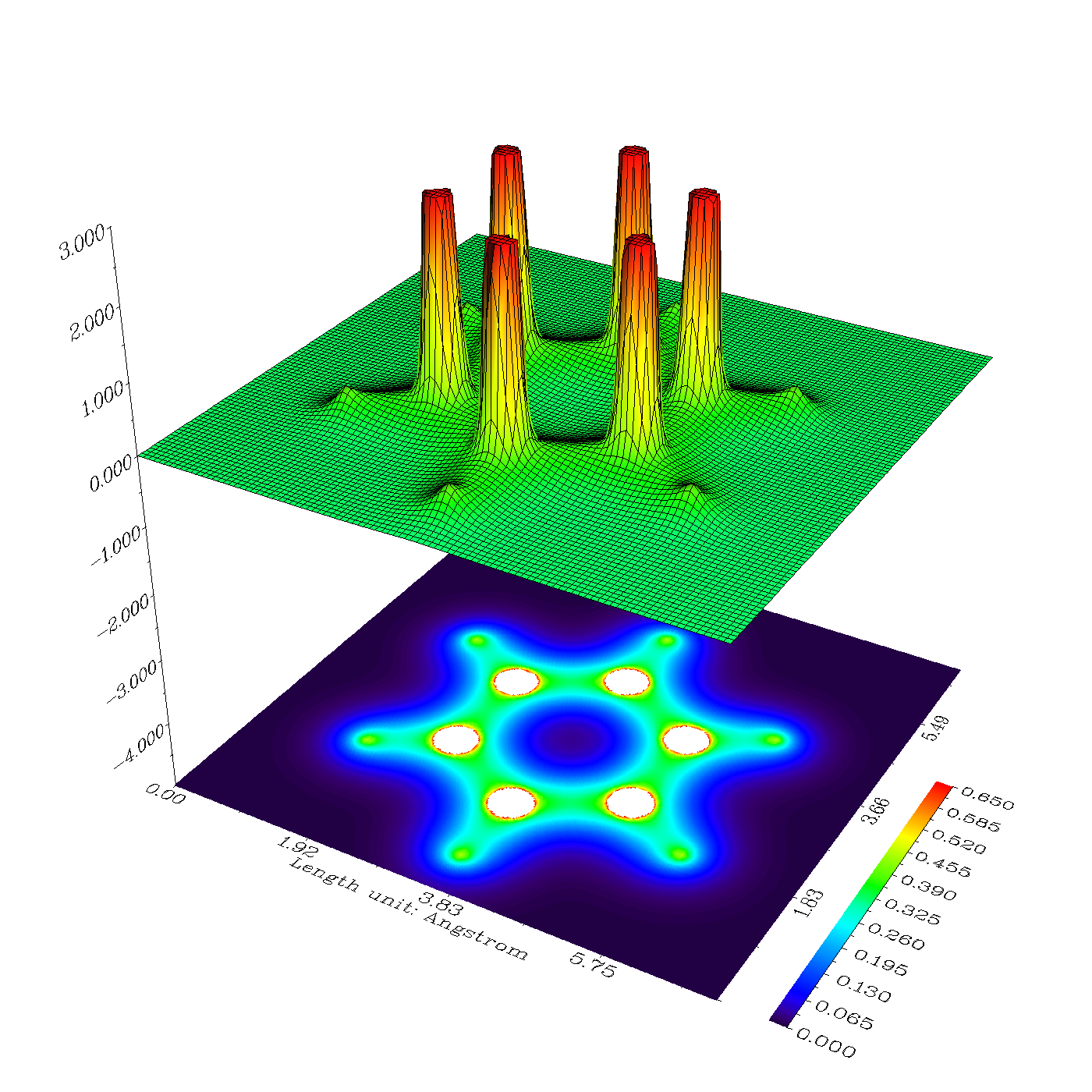
Electron density plays a crucial role in understanding the behavior and properties of atoms and molecules in both chemistry and physics. It refers to the distribution of electrons within an atom or molecule, providing valuable insights into its electronic structure. By studying electron density, scientists can gain a deeper understanding of various phenomena, such as chemical bonding, atomic structure, and quantum mechanics.
Electron Density Phantom
In quantum mechanics, the concept of electron density is closely related to atomic orbitals and electron configuration. Atomic orbitals are regions of space where electrons are most likely to be found. The electron density of an atom is determined by the wave function, which is a mathematical description of the electron‘s behavior. The wave function is obtained by solving the Schrödinger equation, a fundamental equation in quantum mechanics.
The electron density phantom is a theoretical construct used to visualize the electron distribution within an atom or molecule. It represents the probability of finding an electron at a particular point in space. The electron density phantom is based on the principles of quantum mechanics and the Pauli exclusion principle, which states that no two electrons can occupy the same quantum state simultaneously.
Electron Density Model
In molecular orbital theory, electron density is used to describe the distribution of electrons in a molecule. It provides information about the bonding and non-bonding interactions between atoms within the molecule. The electron density model allows scientists to predict and analyze the properties of molecules, such as their stability, reactivity, and spectroscopic behavior.
Chemical bonding is a fundamental concept in chemistry, and electron density plays a crucial role in understanding the nature of chemical bonds. By analyzing the electron density distribution, scientists can determine the strength and type of chemical bonds present in a molecule. This information is essential for studying the structure and properties of various compounds.
Showing Electron Density in Pymol
Pymol is a popular software tool used by scientists to visualize and analyze molecular structures. It allows researchers to display electron density maps, which provide a visual representation of the electron distribution within a molecule. By using Pymol, scientists can explore the electron density of different molecules and gain insights into their electronic structure.
The electron density maps generated in Pymol are based on density functional theory (DFT), a computational method widely used in quantum chemistry. DFT calculates the electron density by solving the Schrödinger equation for the system of interest. The resulting electron density map can be displayed in Pymol, allowing scientists to visualize and analyze the electron distribution in three dimensions.
Miscellaneous Concepts in Electron Density
In the field of quantum mechanics, electron density plays a crucial role in understanding various aspects of atomic and molecular systems. It provides valuable insights into the distribution of electrons within an atom or molecule, which in turn influences its chemical properties. Let’s explore some miscellaneous concepts related to electron density.
Is Electron Density and Current Density the Same?
No, electron density and current density are not the same. Electron density refers to the probability of finding an electron at a specific point in space. It is represented by the square of the wave function, which describes the behavior of an electron in terms of its position and energy. On the other hand, current density refers to the flow of electric charge per unit area. It is related to the movement of electrons in a conductor under the influence of an electric field.
What Happens When Electron Density Increases?
When electron density increases, several interesting phenomena occur. Firstly, an increase in electron density leads to stronger electrostatic interactions between electrons, which affects the stability of atoms and molecules. This can influence chemical bonding and the overall reactivity of a system. Additionally, higher electron density can result in a stronger electron-electron repulsion, leading to a greater energy cost for adding additional electrons.
Furthermore, an increase in electron density can affect the shape and size of atomic orbitals. The distribution of electrons around an atomic nucleus determines the shape of the electron cloud, which in turn determines the shape of the atomic orbitals. Changes in electron density can alter the energy levels and spatial arrangement of these orbitals, influencing the electronic structure of atoms and molecules.
Is Voltage Electron Density?
No, voltage is not directly related to electron density. Voltage, also known as electric potential difference, is a measure of the electric potential energy per unit charge. It represents the force that drives the movement of electric charges in a circuit. On the other hand, electron density refers to the concentration of electrons in a given region of space. While voltage can influence the movement of electrons, it is not a direct measure of electron density.
Electron density plays a significant role in determining the chemical reactivity, stability, and bonding patterns of substances. It helps in predicting the shape and size of molecules, as well as their interactions with other molecules.
Furthermore, electron density is also used in computational chemistry to model and simulate chemical reactions. It provides valuable information about the charge distribution and electron cloud of a system.
Overall, the study of electron density is essential for advancing our understanding of the microscopic world and its impact on the macroscopic properties of matter.
Frequently Asked Questions
What is electron density?
Electron density refers to the measure of the probability of an electron being present at a specific location. In quantum mechanics, it’s derived from the absolute square of the wave function obtained from the Schroedinger equation. Electron density is crucial in understanding atomic structure, chemical bonding, and electron configuration.
How to show electron density in PyMOL?
PyMOL is a software that visualizes molecular structures. To show electron density, first, load the structure file and the electron density map. Then, use the ‘isomesh’ command followed by the desired mesh name, map name, and contour level. Adjust the visualization as needed.
What are electron density units?
Electron density is typically measured in electrons per cubic angstrom (e/ų). This unit is used in quantum chemistry, specifically in density functional theory, to represent the probability density of electrons in a given volume.
What is electron density of states?
Electron density of states refers to the number of electron states per interval of energy at a given energy level that are available to be occupied. This concept is significant in quantum physics and molecular orbital theory.
How to find electron density?
Electron density can be determined using the wave function derived from the Schroedinger equation. The square of the absolute value of the wave function gives the electron density. Computationally, software like Gaussian or PyMOL can be used to calculate and visualize electron density.
What is electron spin density?
Electron spin density refers to the spatial distribution of electrons considering their spin state. This is important in quantum chemistry and is dictated by the Pauli exclusion principle, stating that no two electrons can have the same set of quantum numbers.
Why is electron density important?
Electron density is important as it provides insight into the electronic structure of atoms and molecules. It aids in understanding chemical bonding, electron configuration, and atomic orbitals. Additionally, electron density is used in density functional theory, a cornerstone of quantum chemistry.
What is an electron density phantom?
An electron density phantom is a tool used in medical physics for calibrating imaging devices like CT scanners. It consists of various materials that mimic the electron densities of different human tissues.
What is total electron density?
Total electron density is the summation of the electron densities from all the electrons present in a given system. It provides a complete picture of the electron cloud or distribution around the atomic nucleus.
How does electron density affect polarity?
Electron density affects polarity by influencing the distribution of electrons in a molecule. If electron density is unevenly distributed, it leads to a polar molecule with a positive end and a negative end. This phenomena is crucial in understanding chemical bonding and molecular orbital theory.
Also Read:
- How to find kinetic energy of electron
- How to find energy of electron
- Electron cloud facts of electron cloud model
- Transmission electron microscope tem
- Electron microscopy imaging techniques
- Is photon an electron
- How to find energy level of valence electrons
- Electron microscope 2
- Electron diffraction
- Cryo electron microscopy

The TechieScience Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the TechieScience.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.
